For many men
testosterone replacement therapy (TRT) represents liberation renewed energy, sharper focus, restored libido, and improved strength. In the United States alone, more than 3 million men are now using injectable testosterone formulations for clinically diagnosed hypogonadism. Yet behind these therapeutic gains hides a crucial question that often goes unaddressed: What happens to fertility?
Understanding testosterone functions, normal ranges, and diagnostic evaluation
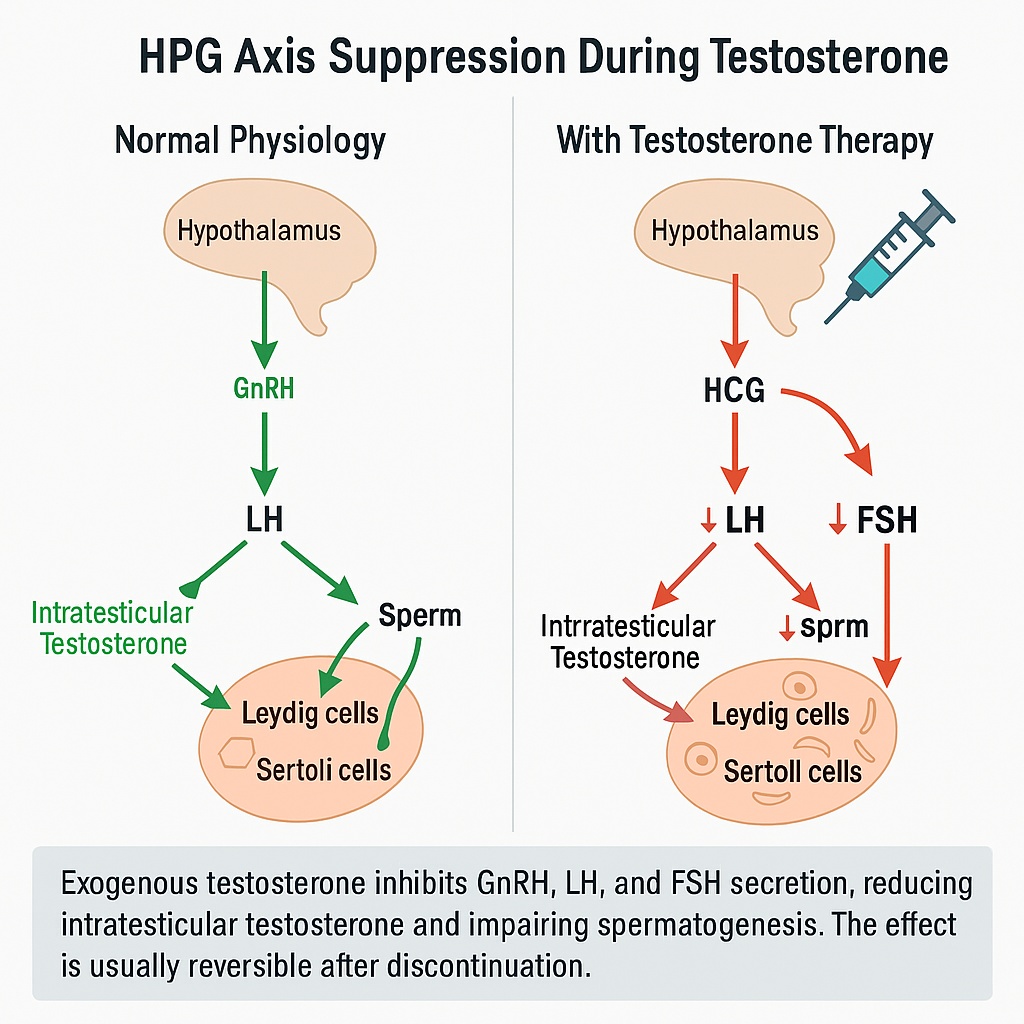
Despite being the hormone of masculinity, exogenous testosterone can paradoxically suppress sperm production. The same mechanism that makes TRT so effective at relieving symptoms also interrupts the hormonal dialogue between the brain and the testes a feedback loop known as the hypothalamic pituitary gonadal (HPG) axis.
In 2025, this issue has moved to the forefront of urology and endocrinology clinics. Fertility preservation has become a major topic as more men seek therapy earlier in life, often before completing their families. The good news is that infertility caused by TRT is usually reversible but recovery requires strategy, time, and sometimes adjunctive treatments. Understanding the physiology of suppression and the window of reversibility is the first step toward protecting spermatogenesis while maintaining hormonal health.
Why Testosterone Therapy Suppresses Spermatogenesis
The normal HPG axis
In a healthy man, the hypothalamus secretes gonadotropin-releasing hormone (GnRH) in rhythmic pulses. This signal prompts the pituitary gland to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
- LH acts on the Leydig cells in the testes, stimulating them to produce testosterone.
- FSH targets the Sertoli cells, which nurture developing sperm cells and sustain the process of spermatogenesis.
Crucially, intratesticular testosterone concentrations are 50- to 100-fold higher than circulating serum levels a gradient essential for sperm development.
What exogenous testosterone does
When injectable testosterone enters the bloodstream, it creates an artificial abundance of androgen. The hypothalamus senses this and shuts down GnRH secretion; the pituitary then reduces LH and FSH output. Without LH, Leydig cells stop producing local testosterone. Without FSH, Sertoli cells lose their stimulation. The testicles, deprived of both signals, gradually shrink, and sperm production slows or stops entirely a process known as secondary hypogonadotropic hypogonadism.
Clinically, most men on standard TRT develop azoospermia or severe oligospermia within three to six months. While this effect is intended in male-contraceptive research, it is an unintended consequence for men who wish to remain fertile.
Reversibility and Recovery Timeline
The suppression of spermatogenesis by testosterone injections is typically reversible, but recovery varies widely. Data from multiple studies, including meta-analyses published in Fertility and Sterility (2023) and Andrology (2024), show that most men regain baseline sperm counts within 6 to 12 months after discontinuing exogenous testosterone.
However, several factors influence recovery speed:
- Duration of therapy: longer exposure leads to slower restoration.
- Age: younger men recover faster due to higher baseline gonadotropin responsiveness.
- Dose and formulation: short-acting injections cause deeper suppression than low-dose or transdermal regimens.
- Baseline fertility status: preexisting testicular dysfunction or varicocele can delay recovery.
Men who have used testosterone for more than two years may require adjunctive therapy with HCG or FSH to re-stimulate endogenous spermatogenesis. This pharmacologic “kick-start” mimics the natural pituitary signals that testosterone suppresses.
Safety monitoring recommendations for men on testosterone therapy
Clinical outlook for 2025
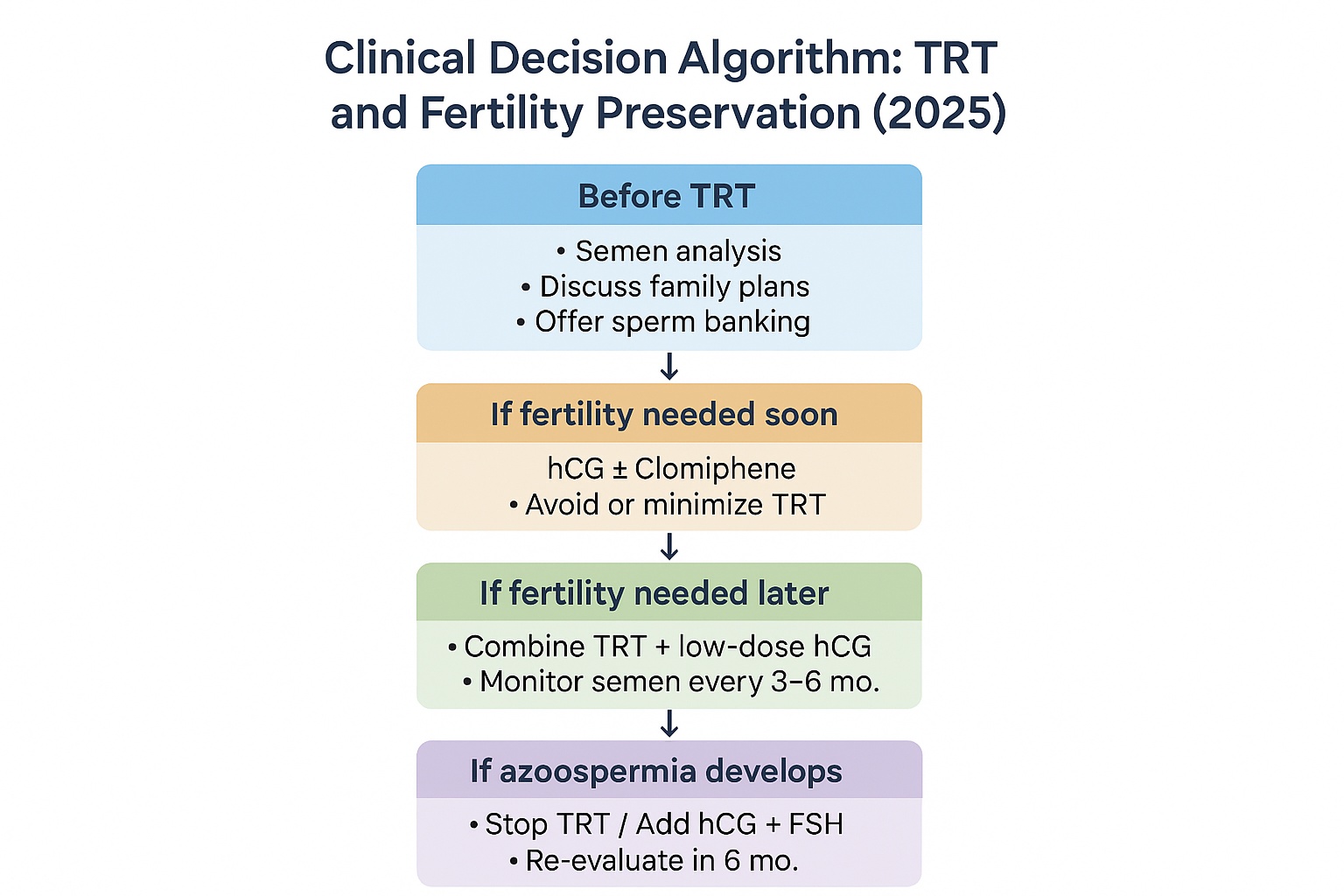
Modern fertility clinics increasingly screen men for family-planning intentions before initiating TRT. Semen analysis and baseline hormone profiling (LH, FSH, total and free testosterone) are recommended for all men of reproductive age. With proper counseling and, if needed, sperm cryopreservation, TRT no longer has to be synonymous with infertility.
The American Urological Association’s 2025 update emphasizes that fertility preservation is part of informed consent for testosterone therapy. Discussing it early allows clinicians and patients to plan interventions such as adding low-dose HCG during TRT to protect spermatogenesis without compromising symptom control.
HCG, FSH, and SERMs: How to Protect Fertility While on Testosterone Therapy
Rationale for combined or alternative therapy
By 2025, clinicians managing men on testosterone replacement therapy are expected to balance two goals restoring androgen levels and preserving fertility. The key lies in mimicking the hormonal signals normally provided by the brain.
When testosterone injections suppress the hypothalamic–pituitary–gonadal (HPG) axis, two main gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) fall dramatically. The testicles, deprived of these signals, stop producing intratesticular testosterone and mature sperm.
Human chorionic gonadotropin (HCG), follicle-stimulating hormone (FSH), and selective estrogen receptor modulators (SERMs) can replace or restore these missing signals, allowing spermatogenesis to continue even during TRT.
Human Chorionic Gonadotropin (HCG)
HCG acts as an LH analog. It binds to LH receptors on Leydig cells, stimulating them to produce testosterone inside the testes maintaining the intratesticular concentration necessary for sperm production.
Clinical studies from Fertility and Sterility (2024) and The Journal of Urology (2025) demonstrate that HCG co-therapy can preserve sperm counts in men receiving exogenous testosterone.
Typical protocols include 500 to 1000 IU of HCG administered subcutaneously two to three times per week. This dose is sufficient to maintain testicular function without significantly increasing serum testosterone beyond target ranges.
In men who already developed azoospermia after long-term TRT, higher “recovery” doses 1500–3000 IU several times weekly may be required for several months before tapering down.
HCG is considered the first-line adjunct for men wishing to remain fertile while continuing TRT.
Follicle-Stimulating Hormone (FSH)
While HCG restores intratesticular testosterone, FSH directly stimulates Sertoli cells, which are essential for sperm maturation. FSH is usually reserved for cases where sperm counts do not recover with HCG alone.
Recombinant FSH (rFSH) is used off-label in the United States for male infertility at doses of 75–150 IU three times per week. Though costly, it significantly improves sperm parameters in men with secondary hypogonadism.
Selective Estrogen Receptor Modulators (SERMs)
Agents like clomiphene citrate and enclomiphene are oral alternatives for men who wish to raise endogenous testosterone without using injections. They block estrogen’s feedback at the hypothalamus, thereby increasing GnRH, LH, and FSH secretion.
Clomiphene is typically used at 25–50 mg every other day, while enclomiphene a more targeted isomer offers improved hormonal stability with fewer side effects. These agents are especially useful in younger men with mild testosterone deficiency who want to maintain natural fertility.
SERMs are also applied during “transition off TRT” to help reactivate the HPG axis and accelerate recovery of sperm production.
Agents Supporting Spermatogenesis During Testosterone Therapy (2025)
| Agent | Mechanism of Action | Clinical Role | Typical Use Case | Evidence Level (2025) |
|---|---|---|---|---|
| HCG | Mimics LH → stimulates Leydig cells → maintains intratesticular T | First-line fertility-preserving adjunct | Men continuing TRT but planning conception | Strong (RCTs, meta-analyses) |
| FSH | Activates Sertoli cells → supports sperm maturation | Add-on if HCG insufficient | Long-term suppression, low sperm count | Moderate |
| Clomiphene / Enclomiphene | ↑ GnRH → ↑ LH/FSH → boosts endogenous T | Alternative to TRT, preserves fertility | Mild hypogonadism, off-TRT recovery | Strong (observational + RCT) |
Caption: HCG remains the cornerstone of fertility preservation in men on testosterone therapy, with FSH and SERMs serving as adjuncts for specific cases.
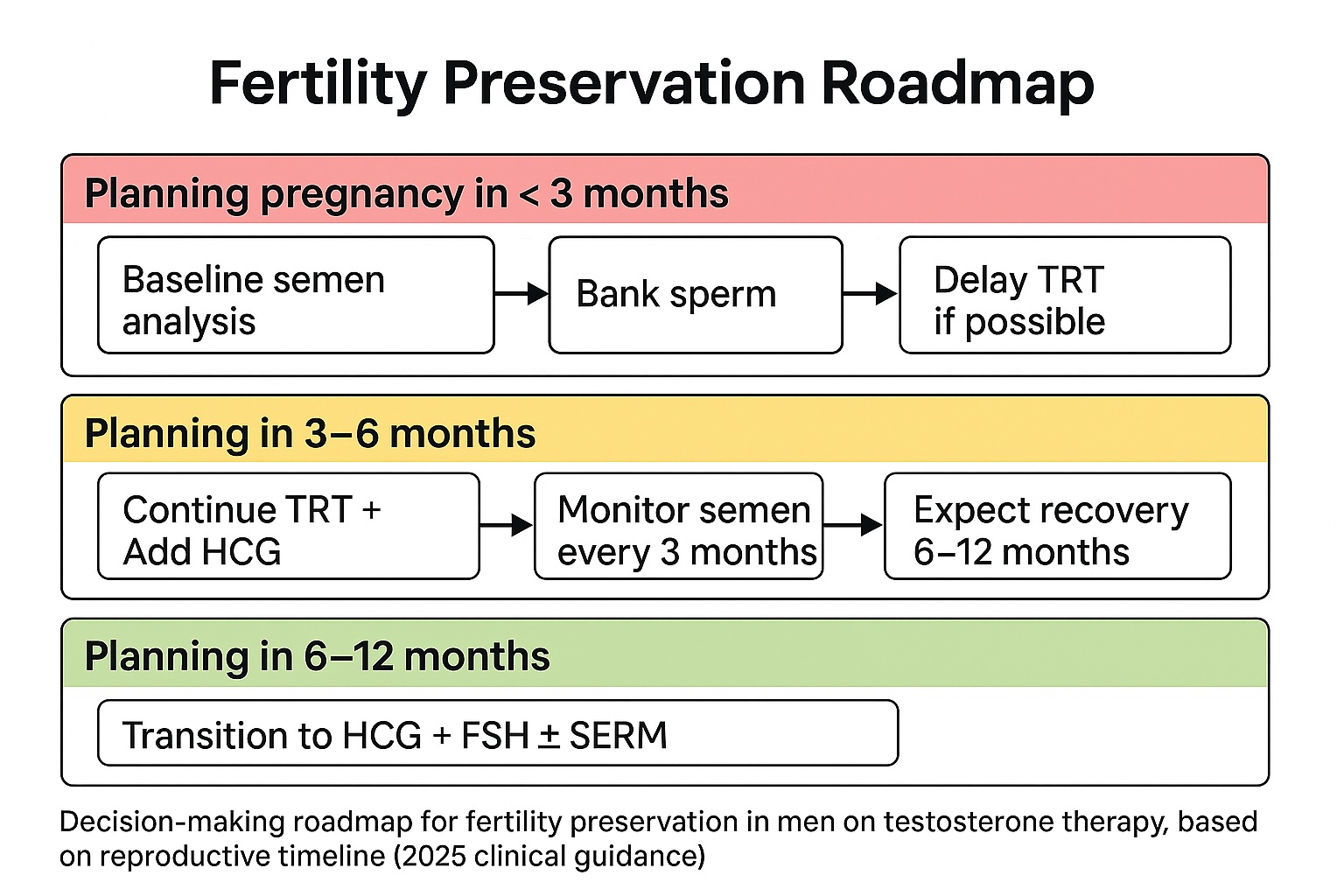
Strategies for Men Planning Pregnancy Within 3–12 Months
Timing matters: defining the reproductive window
Fertility planning must start before the first injection. For men anticipating pregnancy within the next year, timing determines the best management pathway. Recovery of spermatogenesis after testosterone therapy follows a predictable timeline but depends on the duration of suppression.
Clinical roadmaps for fertility preservation
Scenario A: Pregnancy desired within 3 months. TRT should be postponed if possible. Baseline semen analysis is mandatory, and sperm banking is strongly advised. Cryopreservation ensures reproductive security even if TRT leads to temporary azoospermia.
Scenario B: Pregnancy desired within 3–6 months. Switch to HCG monotherapy or combine low-dose HCG with reduced-dose TRT. This preserves testicular stimulation while maintaining serum testosterone levels sufficient to relieve hypogonadal symptoms.
Scenario C: Pregnancy desired within 6–12 months.
Gradually taper testosterone, continue HCG ± FSH, and add a SERM to reactivate endogenous pituitary output. Spermatogenesis recovery typically begins after three months and reaches functional fertility levels by six to twelve months.
Clinicians should reassess sperm parameters every three months and modify therapy based on progress.
Role of fertility specialists
Collaboration between endocrinologists, urologists, and fertility specialists is critical. Personalized hormonal protocols and clear communication of timelines prevent frustration for couples trying to conceive.
The goal is not simply to restore testosterone numbers but to maintain both hormonal balance and the potential for natural conception.
Baseline Semen Testing and Sperm Banking: Planning Fertility Before TRT
Before starting testosterone injections, a man’s reproductive baseline should be objectively assessed. The cornerstone of this evaluation is the semen analysis — a simple yet powerful tool that reveals sperm concentration, motility, and morphology. Even in asymptomatic men, subclinical infertility is not rare; nearly 10–15% of hypogonadal patients present with low baseline sperm counts prior to therapy.
Why semen testing matters
Testosterone therapy can mask underlying testicular dysfunction. Measuring sperm quality beforehand allows clinicians to distinguish preexisting infertility from therapy-induced suppression. It also informs whether sperm banking should be recommended.
A 2024 multicenter study from the American Society for Reproductive Medicine (ASRM) found that 38% of men initiating TRT expressed future family-planning intentions, yet only 9% received fertility counseling. By 2025, U.S. endocrinology and urology guidelines clearly recommend that cryopreservation be discussed before any exogenous testosterone is prescribed.
Sperm banking in the United States (2025)
Cryopreservation is now widely accessible across fertility centers.
- Cost: roughly $500–$900 for collection and analysis, plus $200–$400 annually for storage.
- Longevity: frozen sperm can remain viable for decades with negligible loss of motility.
- Indications: any man under 45 beginning testosterone therapy who may desire children within the next 10 years.
Discussing this option early ensures that fertility remains under the patient’s control rather than therapy’s.
Clinical Scenarios: Balancing Hypogonadism and Fatherhood Goals
Case 1. Secondary hypogonadism with immediate fertility desire
A 32-year-old man presents with fatigue and low libido, diagnosed with secondary hypogonadism. His partner plans pregnancy within 6 months.
Management: Avoid TRT; initiate HCG 1500 IU three times per week ± Clomiphene 25 mg every other day. This restores intratesticular testosterone and normal sperm concentration within 3–4 months.
Case 2. Patient already on TRT for >2 years
A 38-year-old man on testosterone cypionate 200 mg weekly, now wishes to conceive. Semen analysis reveals azoospermia.
Management: Discontinue TRT → Start HCG 3000 IU + rFSH 150 IU thrice weekly for 6 months. Add Clomiphene to stimulate pituitary recovery. Expected sperm return within 6–9 months.
Case 3. Combined hypogonadism with unknown fertility status
A 45-year-old man with borderline primary and secondary hypogonadism, no children yet.
Management: Obtain baseline semen analysis before any therapy. Offer sperm banking. If symptomatic, combine low-dose TRT + HCG (500 IU twice weekly). Monitored PSA, hematocrit, and semen every 3 months.
Clinical message
Fertility and hypogonadism are not mutually exclusive conditions. A well-designed protocol can maintain both normal serum testosterone and active spermatogenesis provided the conversation starts early.
FAQ: Fertility and Testosterone Injections (2025 Update)
Can I stay fertile while taking testosterone?
Yes, with appropriate co-therapy (usually HCG). Many men maintain sperm production while on TRT when monitored correctly.
How long does it take to regain sperm after stopping testosterone?
Average recovery occurs in 6–12 months, but may take up to 18 months in long-term users.
Does HCG really help?
Yes. HCG acts like LH, preserving intratesticular testosterone even when pituitary output is suppressed.
Should I bank sperm before starting testosterone?
If there’s any chance of wanting children in the future, absolutely yes. Cryopreservation is reliable insurance against therapy-induced infertility.
Can Clomiphene replace testosterone therapy?
In mild hypogonadism, yes it can raise natural testosterone while maintaining fertility. However, it may not fully relieve symptoms in severe cases.
References
- Endocrine Society. Testosterone Therapy in Men With Hypogonadism: Clinical Practice Guideline. (2018, updated).
- American Urological Association (AUA). Evaluation and Management of Testosterone Deficiency (Guideline 2024).
- American Society for Reproductive Medicine (ASRM). Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline.
- Preserving fertility in the hypogonadal patient: an update. (PMC free article).
- Management of Male Fertility in Hypogonadal Patients on Testosterone Therapy. MDPI Journal.
- Human chorionic gonadotropin (HCG) / Male Infertility Guide.
